Outcomes of Haematopoietic Stem Cell Transplantation in Beta Thalassemia Major with Fully Matched Parents as Donor
By Asghar Ali Kerio, Nighat Shahbaz, Tariq Azam Khattak, Tariq Ghafoor, Muhammad Farhan, Hashim KhanAffiliations
doi: 10.29271/jcpsp.2024.03.279ABSTRACT
Objective: To determine the outcome of beta thalassemia major (BTM) patients undergoing haematopoietic stem cells (HSCT), with fully matched parents as donors vs. matched sibling donors (MSD).
Study Design: Observational Study.
Place and Duration of the Study: Department of Clinical Haematology and Bone Marrow Transplantation Centre, Rawalpindi, Pakistan, from January 2013 to July 2023.
Methodology: Group A consisted of BTM patients who underwent HSCT with fully matched siblings as donors, and Group B consisted of BTM patients who underwent HSCT with fully matched parents as donors. Study data included the age and gender of both recipients and donors, source and dose of stem cells infused, and stage and grades of acute and chronic graft versus host disease (GvHD). All patients received Myeloablative conditioning regimen (MAC). Data were collected to assess patients’ demographics, response to HSCT, remission rate, disease free survival (DFS), relapse, and GvHD free survival (GRFS), and overall survival (OS).
Results: The mean age of the 54 patients was 5.90 ± 3.29 years. The mean TNC and CD34 doses were 4.99 + 1.13 and 5.42 + 3.70, respectively. Mean time for neutrophil engraftment in both groups was 14.88 + 4.51 days and platelets engraftment was 23.0 + 5.35 days. Most common cause of death was neutropenic sepsis followed by aGVHD. Seven patients had graft rejection. There was no significant association found between graft rejection with donor relation though graft rejection was higher in OS in this study was 70.4%. OS was equal in both groups. Disease free survival was superior in MSD (63%) than parent group (57.7%).
Conclusion: Allogenic bone marrow transplantation with parents as donors in BTM patients yields outcomes comparable to those with matched sibling donors. This finding is especially relevant in regions like Pakistan, where donor registries and high-resolution HLA typing may be limited.
Key Words: Beta thalassemia major, Haematopoietic stem cell transplant, Post-transplant outcome.
INTRODUCTION
Beta-thalassemia major (BTM) is a hereditary condition in which beta-globin chain is reduced or absent.1 Regular blood transfusions are cornerstone of medical care along with iron chelation therapy to prevent tissue damage brought on by transfusion related iron overload.2 In developing countries, the mean life is 20 years due to unavailability of standard treatment options or due to non-affordability.2
Allogeneic haematopoietic stem cell transplantation (Allo-HSCT) from a human leukocyte antigen (HLA) matched family donor has revolutionised the prognosis of patients with homozygous beta-thalassemia.3
It is the sole curative therapeutic option because Allo-HSCT has reported 91% thalassemia-free survival at an early stage of the disease with a mortality risk of ~8%. Since 1982, when the successful HSCT for thalassemia major was carried out, more than 1500 transplants have been completed globally.4 According to published data, overall survival (OS) is over ~80% and event-free survival (EFS) rates is close to 70%.5
Age, stem cell source, histocompatibility, and conditioning regimen are some of the variables that may have an impact on the results of HSCT in BTM.6 HLA mismatch is thought to be the main risk factor for the development of graft versus host disease (GvHD). Other risk variables include the recipient’s CMV viral seropositivity, recipient and donor age, recipient and donor gender mismatch, GvHD prophylaxis, graft type, and TNC and CD34 dose. The scarcity of appropriate donors is one of the biggest obstacles to bone marrow transplantation. Fully matched sibling donors (MSD) have superior transplant results.7 Many countries like Pakistan lack the facility of donor registries and the high cost of searching for unrelated donors make it practically impossible. In Pakistan, patients who do have not MSD, fully matched parents are the only option for bone marrow transplantation.8 The objective of this research was to report the outcome of BTM patients undergoing HSCT, with fully matched parents as donor vs. MSD along with the identification of risk factors for the poor outcomes.
METHODOLOGY
All BTM patients who underwent fully matched HSCT at Bone Marrow Transplant Centre (AFBMTC), Rawalpindi, Pakistan, from January 2013 to February 2023. Total 386 patients underwent bone marrow transplants. Fifty-four patients were included in the final analysis. These patients were divided into two groups. Group A consisted of BTM patients who underwent HSCT with fully matched siblings as donors, and Group B consisted of BTM patients who underwent HSCT with fully matched parents as donors. Ethical guidelines were followed by getting the participant’s parents or guardians informed consent, and official approval was obtained from the institutional review board (IRB#008/AFBMTC/Approval/2021). Using Hb electrophoresis and/or PCR diagnosis for BTM was made. Age, gender, source, and dosage of infused stem cells as well as stages and grades of acute and chronic GvHD were all included in the study data. All patients underwent the Myeloablative conditioning regimen (MAC) which included Fludarabine (Flu), Busulphan (BU), and Cyclophosphamide (Cy) with or without TG. A clinical evaluation of GvHD affecting the skin, gastrointestinal tract, and liver was done using Glucksberg-Seattle criteria. The records of patients with insufficient data and those who underwent a second transplant were excluded.
Information was gathered to evaluate patient characteristics, HSCT response, disease-free survival (DFS), relapse, GVHD-free survival (GRFS), and overall survival (OS). Statistical Package for Social Sciences (SPSS) 25 was used to analyse data. Study produced a variety of variables and among them, frequencies and percentages were determined for categorical and mean, median and standard deviation for continuous data. The link between GvHD and post-transplant complications with Donor-relationship was assessed at univariable analysis using the Pearson Chi-square test and OS was computed using Kaplan-Meier test with statistically significant p-value = 0.05 with 95% confidence interval.
RESULTS
Out of 54 participants, 33 (61.1%) were males and 21 (38.9%) were females. The mean age of the at the time of transplant was 5.90 ± 3.29 years. patients. Bone marrow was the main source used for the collection of stem cells from the donors. The mean TNC and CD34 doses infused into the patients was 4.99 + 1.13 and 5.42 + 3.70, respectively. The mean time for granulocyte recovery in both groups was 14.88 + 4.51 days, whereas the mean time for a self-sustained platelet recovery was 23.0 + 5.35 days. Thirteen (24.1%) patients had major ABO mismatch, 8 (14.8%) had minor ABO, and 33 (61.1%) had no ABO mismatch. Twenty-nine (53.7%) patients were younger than 5 years, 17 (31.5%) were between 5-10 years and 8 (14.8%) were older than 10 years. Out of the 52 donors, 42 (77.8%) were Thalassemia trait. Patients and donor characteristics are given in Table I.
Table I: Demographics of the patients and donors.|
|
Patient |
Donor |
||
|
|
Frequency (n) |
Percentage (%) |
Frequency (n) |
Percentage (%) |
|
Age (Mean + S.D) |
5.90 ± 3.29 years |
9.130 ± 8.13 years |
||
|
RCC Transfusion |
||||
|
Less than 50 |
34 |
63 |
|
|
|
50-100 |
13 |
24.1 |
||
|
More than 100 |
7 |
13 |
||
|
Iron Chelation |
||||
|
Regular |
8 |
14.8 |
|
|
|
Irregular |
46 |
85.2 |
||
|
Ferritin |
||||
|
<2000 ng/ml |
28 |
52 |
|
|
|
>2000 ng/ml |
26 |
48 |
||
|
Organomegaly |
||||
|
Liver |
|
|||
|
Less than 2 cm |
19 |
35 |
||
|
2-5 cm |
33 |
61 |
||
|
More than 5 cm |
2 |
4 |
||
|
Spleen |
||||
|
Non-palpable |
35 |
65 |
|
|
|
Palpable |
19 |
35 |
||
|
Pesaro Class |
||||
|
Class I |
8 |
13 |
|
|
|
Class II |
15 |
27.8 |
||
|
Class III |
31 |
57.4 |
||
|
Donor Relation with Patient |
||||
|
Brother |
|
8 |
14.8 |
|
|
Sister |
19 |
35.2 |
||
|
Father |
11 |
20.6 |
||
|
Mother |
16 |
29.6 |
||
Table II: Relationship of donor status with GvHD.
|
aGVHD |
- |
cGVHD |
- | ||||
|
Yes |
No |
Yes |
No |
- | |||
|
n (%) |
n (%) |
p-value |
n (%) |
n (%) |
p-value |
||
|
Relation of Donor |
- | - | - | - | - | - | |
|
Parents |
- |
13 (24.0) |
14 (26) |
0.009 |
2 (7) |
25 (93) |
0.5 |
|
Sibling |
- |
22 (40.7) |
5 (9.4) |
- |
1 (4) |
26 (96) |
- |
|
Relation of Donor |
- | - | - | - | - | - | |
|
Sister |
- |
6 (75) |
2 (25) |
0.035 |
0 (0) |
19 (100) |
0.54 |
|
Brother |
- |
16 (94) |
1 (6) |
- |
1 (12.5) |
17 (87.5) |
- |
|
Father |
- |
5 (45.5) |
6 (54.5) |
- |
1 (9) |
10 (91) |
- |
|
Mother |
- |
8 (61.5) |
5 (38.5) |
- |
1 (6) |
15 (94) |
- |
|
Donor Gender Mismatched |
- |
- |
- |
- |
- |
- |
|
|
Female to Male |
16 (64) |
9 (36) |
0.9 |
0 (0) |
25 (100) |
0.09 |
|
|
No Mismatch |
19 (65.5) |
10 (34.5) |
-- |
3 (10) |
26 (90) |
-- | |
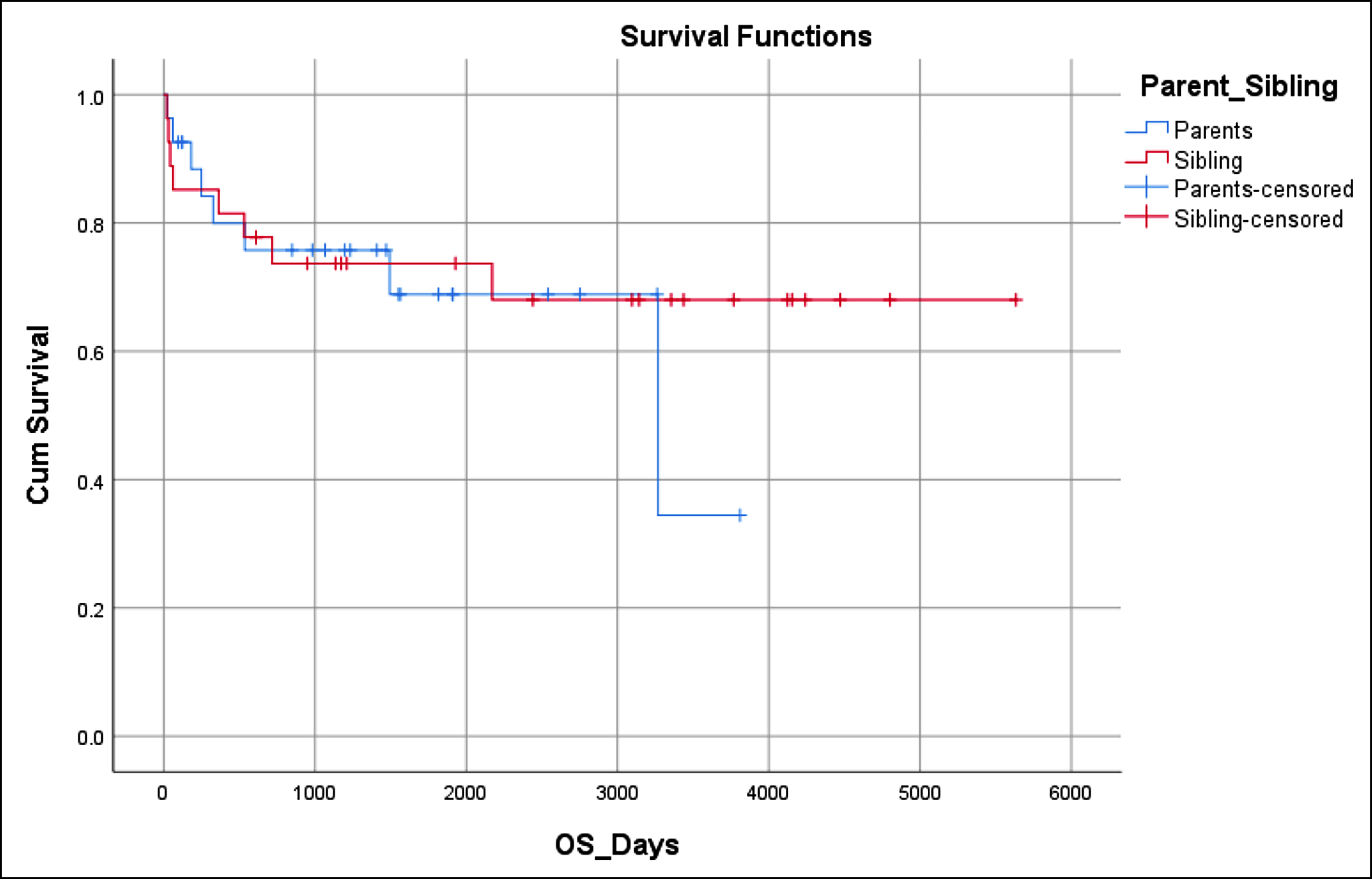 Figure 1: Overall survival (70.4%).
Figure 1: Overall survival (70.4%).
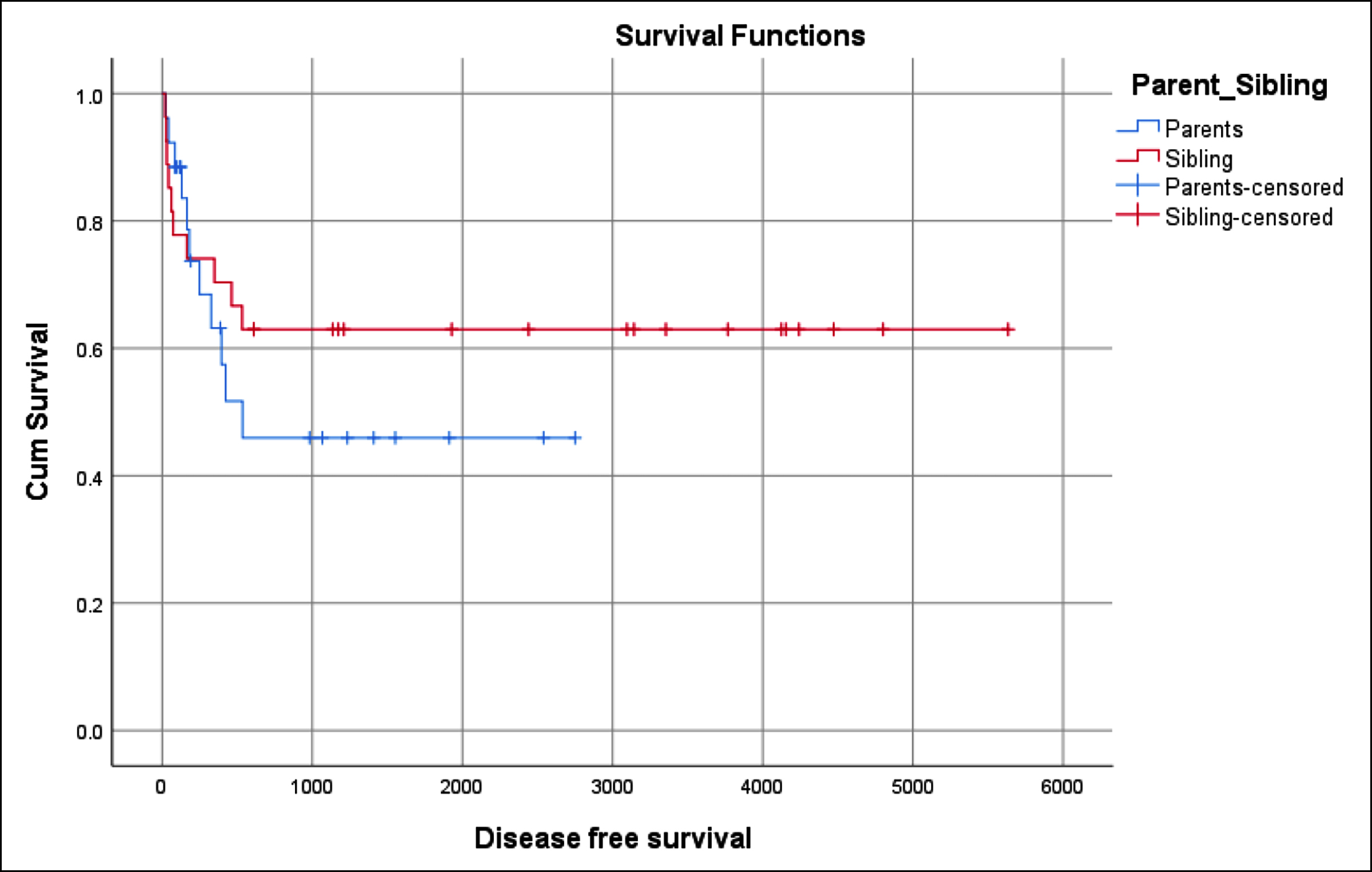 Figure 2: Disease free survival (60.4%).
Figure 2: Disease free survival (60.4%).
Patients were analysed for immediate posttransplant complication out of which neutropenic infections were the most commonly encountered complication i.e. 94.4% (n=51) patients. Neutropenic infections were treated aggressively following the guidelines of neutropenic sepsis. The second most immediate posttransplant complication was cyclosporine-induced hypertension for which patients require introduction of anti-hypertensive. Mucositis was more common in the parent group (n=16, 52%) than the sibling group (n=15, 48%) and this was not statistically significant (p=0.7). Veno-oclusive disease was more common in the parent group (n=6, 75%) than the sibling group (n=2, 25%) with p-value of 0.12. CMV reactivation was observed more in parent group (n=12, 75%) than sibling group (n=4, 25%) and this was statistically significant (p=0.01).
Acute GVHD (aGVHD) was more common in the sibling group and showed statistically significant (p=0.009). aGVHD also showed statistically significant correlation with the relation of donor (p=0.035). Gender mismatch showed no statistically significant relation with aGVHD. However, aGVHD was more common in females than males gender. Details of aGVHD and cGVHD are given in Table II. CMV reactivation was more common in the parent group (85.1%) than in the sibling group (77.7%).
Out of 54 patients, 16 (29.6%) patients died, eight in each group. Most common cause of death was neutropenic sepsis followed by aGVHD. Seven (12.9%) patients had graft rejection. There was no significant association found between graft rejection with donor relation though graft rejection was higher in parents (7.4%) than in siblings (3.7%) (p=0.38).
OS in this study was 70.4% (Figure 1). OS was equal in both groups. DFS in these patients was 60.4% (Figure 2). DFS was superior in MSD (63.0%) than the parent group (57.7%). The impact of the donor’s relation with the patient on the outcomes of HSCT was analysed. Different outcomes had statistical significant impact with the relation of the donor to the patient. The outcome which was analysed included achieving successful engraftment of both neutrophils and platelets, development of mucositis, haemorrhagic cystitis, infections, CMV reactivation, aGVHD, cGVHD, day 100 mortality and long-term OS and DFS. Also, the impact of the donor relation on the fate of graft was analysed. None of the parameters was statistically affected by the donor’s relation except the development of aGvHD and CMV reactivation.
DISCUSSION
Many countries like Pakistan lack the facility of donor registries as high cost of searching for unrelated donors make it practically impossible. In Pakistan, patients who not have MSD, fully matched parents are the alternate donor option for bone marrow transplantation.
Mean age of patients in this study was 5.90 ± 3.29 years. Aydınok et al. from Turkey reported mean age at the time of HSCT in patients with BTM was reported as 6.6 years.9 Mean stem cells dose (CD34 and TNC) in study was 4.99 + 1.13 and 5.42 + 3.70, respectively. Gazievet reported a similar dose of stem cells in BTM patients 4.3 and 6.3, respectively.10 Mean time for neutrophile and platelets engraftment was 14.88 + 4.51 days, and 23.0 + 5.35, respectively. However, Anurathapan et al. from Thailand reported mean of neutrophils and platelets engraftment was 14 and 20 days respectively.11 Infection (94.40%) was the most common immediate post-transplant complication followed CSA-induced hypertension (85.20%). CMV Reactivation was more common in the parent group and this was statistically significant (p=0.01). In contrast, a recent study from Thailand reported mucositis as the most common immediate posttransplant complication followed by VOD. This study did not correlate immediate complications with relation of donor.12
Acute GVHD in this study was more common in the sibling group with statistical significance (p =0.009). Acute GvHD was more common in the group of female donors to male recipients but this was statistically not significant (p=0.1). Chronic GvHD was reported in three cases (5.5%), two were from sibling and one was from the parent group with no statistically significant correlation with donor gender (p=0.5). The authors did not find any national or international data that directly compared the outcomes of allogeneic bone marrow transplantation utilising fully matched parents as donors versus matched sibling donors. However, Swaminathan et al. from India reported that aGVHD was more common in match unrelated donors (MUD) (60%) than MSD (20%).13 This was statistically significant (p=0.001). Swaminathan et al. report-ed higher incidence of cGVHD in the MUD group than MSD and showed statistically significant relationship (p=0.003).13
OS was equal in both groups (70.4%). This was not statistically significant (p=0.11). DFS in these patients was 60.4%. DFS was superior in MSD (63.0%) than the parent group (57.7%). Caocciet from Italy reported superior OS (78%) in MSD than unrelated donors (57.6%). DFS was also superior (76.7%) in the MSD group. This was not statistically significant (p = 0.014).14 Graft rejection in this study was reported in six patients (11.8%). Graft rejection was higher in the parent group (7.4%) than in the sibling group (3.7%). This was not statistically significant (p=0.38). Korula et al. reported a high incidence of graft rejection in match related donors (20.8%) than MSD (5.2%) and was statistically significant (p=0.027).15 In this study, total 14 (25.9%) patients died, 8 in the sibling group and 6 in the parent group. Most common cause of death was neutropenic sepsis followed aGVHD. However, Korula et al. reported higher incidence of death in match related donors (20.8%) than MSD (15.8%).15 The main limitation of the study was small number of patients.
CONCLUSION
In summary, this research demonstrates that allogenic bone marrow transplantation with parents as donors in BTM patients yields outcomes comparable to those with matched sibling donors. This finding is especially relevant in regions like Pakistan, where donor registries and high-resolution HLA typing may be limited. In other countries or regions with more advanced transplant infrastructure, the findings may not necessarily apply, and other donor options could be considered.
ETHICAL APPROVAL:
Ethical approval was obtained from the institutional review board of Armed Forces Bone Marrow Transplantation Centre. (IRB#008/AFBMTC/Approval/2021).
PATIENTS’ CONSENT:
Informed consent was obtained from patients to publish the data.
COMPETING INTEREST:
The authors declared no conflict or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
AUTHORS’ CONTRIBUTION:
AAK: Acquisition, drafting the work, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
NS: Conception and design of the work.
TKA, MF: Revising it critically for important intellectual content.
TG: Analysis and interpretation of data for the work.
H: Final approval of the version to be published.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Needs T, Gonzalez-Mosquera LF, Lynch DT. Beta thalassemia. 2013. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531481/.
- Farmakis D, Porter J, Taher A, Domenica Cappellini M, Angastiniotis M, Eleftheriou A. 2021 thalassaemia international federation guidelines for the management of transfusion-dependent thalassemia. Hemasphere 2022; 6(8): e732. doi: 10.1097/HS9.0000000000000732.
- Calle AL. Hematopoietic stem cell gene therapy for the treatment of beta-hemoglobinopathies [Doctoral dissertation]. Eberhard Karls Universität Tübingen; 2022. doi:10. 15496/publikation-52138.
- Alonso L, González-Vicent M, Belendez C, Badell I, Sastre A, Rodríguez-Villa A, et al. Hematopoietic stem cell transplantation in pediatric patients with β-thalassemia and sickle cell disease: An experience of the Spanish Working Group for Bone Marrow Transplantation in Children (GETMON). Med Clin (Barc) 2019; 152(4):135-40. doi: 10.1016/j.medcli.2018.05.013.
- Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: Data from the UK thalassaemia register. Lancet 2000; 355(9220):2051-2. doi: 10.1016/ S0140-6736(00)02357-6.
- Al Malki MM, Tsai NC, Palmer J, Mokhtari S, Tsai W, Cao T, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv 2021; 5(12):2650-59. doi: 10.1182/bloodadvances.2021004192.
- Ghavamzadeh A, Nasseri P, Eshraghian MR, Jahani M, Baybordi I, Nateghi J, et al. Prognostic factors in bone marrow transplantation for beta thalassemia major: Experiences from Iran. Bone Marrow Transplant 1998; 22(12): 1167-9. doi: 10.1038/sj.bmt.1701509.
- Pruitt A, Gao F, De Togni E, Cochran H, Godbole S, Slade M, et al. Impact of donor age and relationship on outcomes of peripheral blood haploidentical hematopoietic cell transplantation. Bone Marrow Transplant 2023; 58(8):855-62. doi: 10.1038/s41409-023-01984-8.
- Zaidi U, Shamsi TS, Farzana T, Ansari SH, Borhani M, Munzir S, et al. Capacity building of stem cell transplantation facilities in Pakistan: Joint efforts of NIBD, government, and private-sector institutions. Blood Adv 2019; 3(Suppl 1):41-4. doi: 10.1182/bloodadvances.2019GS121552.
- Aydınok Y, Oymak Y, Atabay B, Aydoğan G, Yeşilipek A, Ünal S, et al. A National registry of thalassemia in Turkey: Demographic and disease characteristics of patients, achievements, and challenges in prevention. Turk J Haematol 2018; 35(1):12-8. doi: 10.4274/tjh.2017.0039.
- Gaziev J, Marziali M, Isgrò A, Sodani P, Paciaroni K, Gallucci C, et al. Bone marrow transplantation for thalassemia from alternative related donors: Improved outcomes with a new approach. Blood 2013; 122(15):2751-6. doi: 10.1182/blood-2013-07-513473.
- Anurathapan U, Hongeng S, Pakakasama S, Songdej D, Sirachainan N, Pongphitcha P, et al. Hematopoietic stem cell transplantation for severe thalassemia patients from haploidentical donors using a novel conditioning regimen. Biol Blood Marrow Transplant 2020; 26(6):1106-12. doi: 10.1016/j.bbmt.2020.01.002.
- Swaminathan VV, Uppuluri R, Patel S, Ravichandran N, Ramanan KM, Vaidhyanathan L, et al. Matched family versus alternative donor hematopoietic stem cell transplantation for patients with thalassemia major: Experience from a tertiary referral center in South India. Biol Blood Marrow Transplant 2020; 26(7):1326-31. doi: 10.1016/j.bbmt. 2020.03.016.
- Caocci G, Orofino MG, Vacca A, Piroddi A, Piras E, Addari MC, et al. Long-term survival of beta thalassemia major patients treated with hematopoietic stem cell transplantation compared with survival with conventional treatment. Am J Hematol 2017; 92(12):1303-10. doi: 10.1002/ajh. 24898.
- Korula A, Fouzia N, Devasia A, Kulkarni UP, Lakshmi KM, Edison ES, et al. Higher Incidence of graft rejection in non-Sibling fully matched related donor stem cell transplants for thalassemia major: A Cautionary Note. Blood 2018; 132:2178. doi: 10.1182/blood-2018-99-117544.